Hybridisation Explained
While we have our regular s, p and d orbitals, we can also have hybrid orbitals, orbitals created from combining a few orbitals together. For example, s orbitals and p orbitals can combine to form a variety of hybrid orbitals, the sp, sp² and sp³ orbitals. Let’s delve more into this!
Why do we bother with hybridisation?
The hybridisation model helps us to explain the shapes of molecules (e.g. tetrahedral, trigonal planar, linear). Without the hybridisation model, we will not be able to account for the shapes around carbon in ethane (tetrahedral), ethene (trigonal planar) and ethyne (linear).
I will explain this as we go, but for now, let’s dive into the details of each type of hybridisation.
sp³ hybridisation
Let’s take for example carbon. Carbon has an atomic number of 6 and therefore an electronic configuration of 1s² 2s² 2p².
Drawing out the orbitals in the valence shell of carbon (without the electrons), we get:
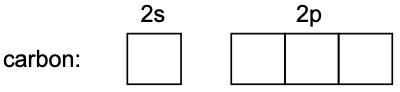
In sp³, the ‘s’ refers to one s orbital and the ‘p³’ refers to three p orbitals. Therefore, one s orbital and three p orbitals combine to form sp³ orbitals.

Note: The number of orbitals used to hybridise = the number of hybrid orbitals formed. Since we used a total of four orbitals (one s + three p) to hybridise, we will also obtain four sp³ hybrid orbitals.
We can visualise the hybridisation through the following diagram:
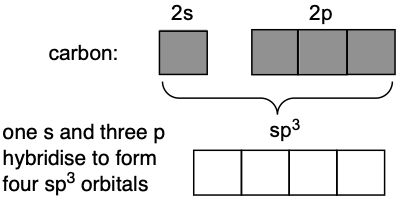
One s orbital and three p orbitals (shaded) hybridise to form four sp³ hybrid orbitals. All orbitals are used up in the hybridisation process.
What do these four sp³ hybrid orbitals look like?
We can draw this out, but first we have to know what a hybrid orbital looks like. A hybrid orbital, regardless of sp, sp² or sp³ hybridised, is made up of a big loop and a tiny loop:

Drawing in four sp³ hybrid orbitals, we get:

© 2012, Jeff Cruzan
This was beautifully drawn, by the computer. But let’s say you are drawing by hand; it would suffice to draw something like that:
How does hybridisation explain shapes?
Notice the way the sp³ orbitals were drawn – in a tetrahedral shape. According to the VSEPR theory, the angle that best minimises repulsion between four bond pairs of electrons is 109.5° (tetrahedral). Therefore, since the four sp³ orbitals contain electrons repelling each other, they arrange themselves in a tetrahedral shape to best minimise repulsion. This explains why molecules like methane are tetrahedral-shaped.

Drawing the orbitals of methane by hand:
The four sp³ orbitals on carbon each form a sigma bond with the 1s orbital of H, forming four sigma bonds.
If you wanted a clearer diagram of the orbitals in methane, here it is!

© 2012, Jeff Cruzan
Sigma bonds have been formed through the head-on overlap of the 1s orbitals of H and the sp³ orbitals of carbon.
What if we didn’t have the hybridisation model? Would we still be able to explain why methane is tetrahedral-shaped? Let’s try reasoning it out! If the carbon in methane was not hybridised, it would still have its original one s and three p orbitals. The three p orbitals (px, py and pz) are 90 degrees to each other, so drawing them in with the s orbital in the center, we would get:

© 2012, Jeff Cruzan
You would not get a tetrahedral shape at all. Instead, the 6 ends of the p orbitals will form an octahedral shape instead, which fails to explain the tetrahedral shape of methane.
Therefore, the hybridisation model is useful in helping us to explain shapes.
sp² hybridisation
In sp², the ‘s’ refers to one s orbital and the ‘p²’ refers to two p orbitals. Therefore, one s orbital and two p orbitals combine to form sp² orbitals.
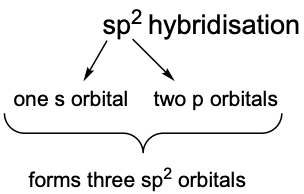
Following the same rule (the number of orbitals used to hybridise = the number of hybrid orbitals formed), since we used a total of three orbitals (one s + two p) to hybridise, we will also obtain three sp² hybrid orbitals.
We can visualise the hybridisation through the following diagram:

One s orbital and two p orbitals (shaded) are hybridised to form three sp² hybrid orbitals. Since one p orbital was unused (unshaded), it remains unhybridised. We call this an “unhybridised p orbital”.
Drawing the three sp² orbitals (blue) and unybridised p orbital (grey):

© 2012, Jeff Cruzan
You may draw the orbitals by hand in this manner:
Notice the way the sp² orbitals were drawn – in a trigonal planar shape. According to the VSEPR theory, the angle that best minimises repulsion between three bond pairs of electrons is 120° (trigonal planar). Therefore, since the three sp² orbitals contain electrons repelling each other, they arrange themselves in a trigonal planar shape to best minimise repulsion. This explains why the shape about carbon in molecules like ethene is trigonal planar.
Note that the unhybridised p orbital (grey) is perpendicular to the three sp² orbitals.
Ethene is an example of a molecule with sp² hybridised carbons. The carbon in ethene has three bond pairs, and thus the shape around it is trigonal planar and the angle about it is 120°.

To draw out the orbitals in ethene, we note the following first:
Sigma bonds are formed from the head-on overlap of hybrid orbitals.
Pi bonds are formed from the side-on overlap of unhybridised p orbitals.
Drawing out the orbitals in ethene:
Don’t forget to label your orbitals and bonds! The head-on overlaps are labelled as sigma bonds and the side-on overlaps are labelled as pi bonds. Note that the pair of dotted-lines between the two carbons represent only one pi bond, not two.
If you wanted a clearer diagram of the orbitals around the carbon in ethene, here it is!

© 2012, Jeff Cruzan
A sigma bond has been formed through the head-on overlap of the sp² orbitals of the two carbons.
sp hybridisation
You get the drift by now.
In sp, the ‘s’ refers to one s orbital and the ‘p’ refers to one p orbital. Therefore, one s orbital and one p orbital combine to form sp orbitals.

Since we used a total of two orbitals (one s + one p) to hybridise, we will also obtain two sp hybrid orbitals.
We can visualise the hybridisation through the following diagram:

One s orbital and one p orbital (shaded) hybridise to form two sp hybrid orbitals. Since two p orbitals were unused (unshaded), we have two unhybridised p orbitals as a result.
Drawing the two sp orbitals (blue and orange) and two unhybridised p orbitals (grey):

© 2012, Jeff Cruzan
The two sp orbitals are 180° to each other, forming a linear shape. Meanwhile, the two unhybridised p orbitals (grey) are perpendicular to each other and the sp orbitals.
You may draw the orbitals by hand in this manner:
Notice the way the sp orbitals were drawn – in a linear shape. According to the VSEPR theory, the angle that best minimises repulsion between two bond pairs of electrons is 180° (linear). Therefore, since the two sp orbitals contain electrons repelling each other, they arrange themselves in a linear shape to best minimise repulsion. This explains why the shape about carbon in molecules like ethyne is linear
Ethyne is an example of a molecule with sp hybridised carbons. The carbon in ethyne has two bond pairs, and thus the shape around it is linear and the angle about it is 180°.

Remember that
Sigma bonds are formed from the head-on overlap of hybrid orbitals.
Pi bonds are formed from the side-on overlap of unhybridised p orbitals.
Drawing out the orbitals in ethyne:
The red pair of p orbitals are perpendicular to the blue pair of p orbitals. The red pair side-on overlaps to form one pi bond while the blue pair side-on overlaps to form another pi bond. A sigma bond is formed from the head-on overlap of the sp orbitals.
Top view of orbitals in ethyne:

© 2012, Jeff Cruzan
Determining hybridisation of carbon in a molecule
To quickly determine the hybridisation of any carbon atom in a molecule, look at the shape about that carbon.
1. Tetrahedral about carbon = sp³ hybridised
2. Trigonal planar about carbon = sp² hybridised
3. Linear about carbon = sp hybridised
Here are some examples:

Examples 1-3 are methane, ethene and ethyne, which I have previously discussed. In methane, the carbon has 4 bond pairs and 0 lone pairs, thus being tetrahedral. Therefore, it is sp³ hybridised. The same idea applies for examples 2 and 3.
(Note that double bonds or triple bonds are still counted as one bond pair)
Examples 4 and 5 can be tricky as they are skeletal structures. All the carbons in example 4 are sp² hybridised, as they all have 3 bond pairs and are therefore trigonal planar. If you were unable to identify 3 bond pairs, you might be forgetting the hydrogens that are not shown in the skeletal structure. Carbon a has one hydrogen attached to it while carbon b has 2 hydrogens attached to it.
Likewise, in the carbocation of example 5, carbon c has one (unshown) hydrogen attached to it, and therefore has 3 bond pairs, becoming trigonal planar and therefore sp² hybridised. (In fact, carbocations are always sp² hybridised as they come from a carbon with 4 bonds losing one bond to have 3 bond pairs.)
Determining hybridisation of heteroatoms (e.g. O, Cl, N) attached to carbon
Many a times we would encounter molecules such as:
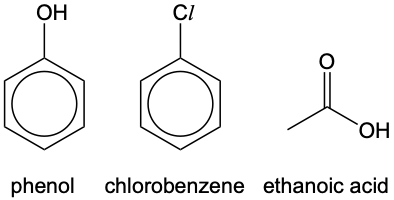
How do we determine the hybridisation of O and Cl in these molecules? We may follow this set of rules:
An atom is also sp² hybridised if it
Contains at least one lone pair of electrons
Is bonded to an sp² hybridised carbon
In phenol, the O contains at least one lone pair of electrons (two) and is bonded to an sp² hybridised carbon of benzene, and is thus sp² hybridised too. Likewise, the Cl in chlorobenzene, having at least one lone pair of electrons (three) and is bonded to an sp² hybridised carbon of benzene, is sp² hybridised too.
Lastly, in ethanoic acid, both Os are sp² hybridised as they have at least one lone pair of electrons and is bonded to an sp² hybridised carbon.

Since the O and Cl in phenol, chlorobenzene and ethanoic acid are all sp² hybridised, they each have a p orbital. The presence of p orbitals allow for delocalisation of electrons to occur (i.e. resonance). Read more about resonance in "Resonance Explained".
One last exception
In phenylamine, one might think that the N is sp² hybridised as it contains a lone pair of electrons and is bonded to an sp² hybridised carbon (following the above rules). However, the N on phenylamine is somewhere between sp² and sp³ hybridised.
I will not get into the details of why this is so, but for this reason, it is wrong to say that N is sp² hybridised and has a p orbital. Instead, we will refer to the orbital on N as “the orbital containing the lone pair of electrons on N".

Comments