If you've ever binge-watched Breaking Bad, you would have witnessed the exhilarating action of Chemistry in many scenes. The protagonist, Walter White is a badass genius who cleverly uses his Chemistry chops to wriggle his way out of some pretty sticky situations, one of which was paying off his medical bills by..... let's just say.... making it big in the pharmaceutical industry. Some of my students have cheekily suggested that I, a high school Chemistry teacher, should learn from him and be a little more entrepreneurial in my aspirations, But I, someone with morals, have decided to stick with my average joe job, one in which I’m being compensated in terms beyond your wildest dreams – if your dreams are super mediocre. Who needs a meth empire when you can revel in the glory of teaching high school Chemistry, right?
In Breaking Bad, we see Walter White and Jesse, Walter’s protégé, stranded in the middle of a desert, stuck in their broken-down RV (recreational vehicle). Dumb Jesse left the car keys in the ignition for two days, draining the RV of its entire battery. To quote Walter, “Is this just a genetic thing with you? Is it congenital? Did your mother drop you on your head when you were a baby?”
Technically, they could trek their way out, but Jesse used up the rest of their drinking water to douse a fire. And not to mention, after camping it out for 3 days in the desert, their bodies were dangerously low on electrolytes. So… what would you do if you were in their shoes? Walter decided – why not build a homemade battery? Watch the following video to see him do just that:
How realistic was this scene?
Ever thought Chemistry could one day save your life? When I first watched this scene I thought, “Genius!”, then on second thoughts, “hold up…really? A few homemade batteries with some cut-up sponges – that’ll do it? What is the minimum voltage required to start a car, or an RV?”
So… how realistic was this scene? Let’s break it down!
From the video, this is what we can gather:
Walter made the
1. Anode – from coins, nuts, bolts, screws that were galvanised with zinc
2. Cathode – from solid mercuric oxide and graphite from the brake pads of the RV
3. Electrolyte – potassium hydroxide (that they were using for their ‘chemistry experiment’)
4. Wire – copper wire
We also know/ can set some conditions in which they built their battery:
Number of cells connected in series: 6 cells
Location: Outskirts of Albuquerque, New Mexico, USA
Usual voltage required to jumpstart an RV: 12V (thanks Google)
Kₛₚ of HgO (s) at the average temperature in the desert: assume 1.204 x 10⁻⁷mol²dm⁻⁶
[Zn²⁺]: assume 3.0 x 10⁻¹⁴ mol dm⁻³ (not mentioned in scene)
Assume 0.1 mol dm⁻³ potassium hydroxide electrolyte (not mentioned in scene)
Knowing these, we can draw a diagram of Walter’s set-up:
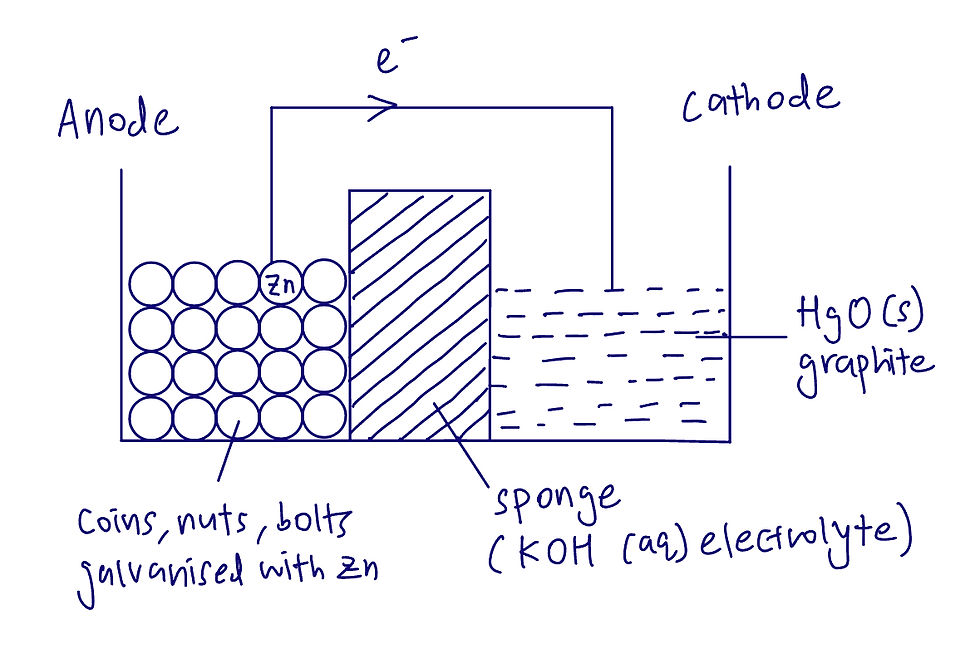
Calculations:
Let’s werk it out. At the anode, we have Zn from the coins, nuts and bolts that gets oxidised to Zn²⁺. At the cathode, we have solid HgO (mercuric oxide), which dissolves in the electrolyte to form Hg²⁺, which then gets reduced to solid Hg.
Anode: Zn²⁺(aq) + 2e⁻ ⇌ Zn(s) E ° = –0.763 V
Cathode: Hg²⁺(aq) + 2e⁻ ⇌ Hg(s) E ° = +0.854 V
Full equation: Zn(s) + Hg²⁺(aq) → Zn²⁺(aq) + Hg(s)
To calculate the voltage (E °cell) generated from one cell, one would usually use the formula:
E °cell = E °cathode – E °anode
However, recall that this formula can only be used at standard conditions (as indicated by °).
Considering that this battery was built in a desert, where the temperature is wonky, we can use the Nernst equation, which calculates Ecell under non-standard conditions.
The Nernst equation for this cell can be written as:
Ecell = E °cell – (9.916 x 10⁻⁵)T log ( [Zn²⁺]/[Hg²⁺] )
Let’s take the average temperature in a desert (in the day) to be 38°C (thanks, Google).
We can calculate E °cell as such: E °cell = E °cathode – E °anode = +0.854 –(–0.763) = +1.617V
Since we have temperature (T), E °cell and [Zn²⁺], all we are left to find is [Hg²⁺].
HgO(s) is sparingly soluble. Assuming the metal oxide is a basic oxide, it dissolves to give hydroxide ions. If we let the solubility of HgO be s mol dm⁻³, we can calculate the equilibrium concentrations of Hg²⁺ and OH⁻ respectively:
HgO(s) + H₂O(l) ⇌ Hg²⁺ (aq) + 2OH⁻ (aq)
Initial conc. / mol dm⁻³ 0 0.1
Change in conc. / mol dm⁻³ +s +2s
Equilibrium conc. / mol dm⁻³ s 0.1 + 2s ≈ 0.1
Since HgO is sparingly soluble, s (solubility) is very small and insignificant compared to the 0.1 mol dm⁻³ of KOH. Since 2s << 0.1, 0.1 + 2s can be approximated to 0.1 mol dm⁻³. Substituting the equilibrium concentrations into Kₛₚ, we get:
Kₛₚ = [Hg²⁺][OH⁻]² = 1.204 x 10⁻⁷
(s)(0.1)² = 1.204 x 10⁻⁷
s = 1.204 x 10⁻⁵ = [Hg²⁺]
Finally! We have everything we need in the Nernst equation. Let’s sub them in:
Ecell = E °cell – (9.916 x 10⁻⁵)T log ( [Zn²⁺]/[Hg²⁺] )
= +1.617 – (9.916 x 10⁻⁵)(38 + 273) lg[(3 x 10⁻¹⁴)/(1.204 x 10⁻⁵)]
= +1.8823V is the voltage produced by one cell of a battery
Since we have 6 cells connected in series, the total voltage produced by the homemade battery is… drumrollsssssss
Total voltage produced = +1.8823 x 6 = +11.29 V
which is less than the 12V required for the RV to work properly.
So there you go, the scene was not as realistic as we hoped it would be. Maybe a better idea would have been asking Walter to use his baldness to generate solar energy instead.

Comments